
ISSN:1692-7273 | eISSN:2145-4507



Effect of Chronic Altitude Hypoxia on Redox Balance in Preadolescents and Adolescents
Efectos de la hipoxia altitudinal crónica sobre el balance Redox de preadolescentes y adolescentes
Efeito da exposição crônica à hipóxia de altitude no equilíbrio oxidante/antioxidante de crianças e adolescentes
Diana Marcela Ramos-Caballero, Pilar Rodríguez-Rodríguez, Erica Mancera-Soto, Sandra Martins, Silvia M. Arribas, José Magalhães, Edgar Cristancho-Mejía
Effect of Chronic Altitude Hypoxia on Redox Balance in Preadolescents and Adolescents
Revista Ciencias de la Salud, vol. 20, no. 3, 2022
Universidad del Rosario
Diana Marcela Ramos-Caballero * diana.ramos@urosario.edu.co
Universidad Nacional de Colombia, Colombia
 Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Pilar Rodríguez-Rodríguez
Universidad Autónoma de Madrid, España
 Contribution: processed and analyzed samples, analyzed data; wrote the manuscript; read and approved the manuscript
Contribution: processed and analyzed samples, analyzed data; wrote the manuscript; read and approved the manuscript
Erica Mancera-Soto
Universidad Nacional de Colombia, Colombia
 Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Sandra Martins
Universidade do Porto, Portugal
 Contribution: processed and analyzed samples, analyzed data; wrote the manuscript; read and approved the manuscript
Contribution: processed and analyzed samples, analyzed data; wrote the manuscript; read and approved the manuscript
Silvia M. Arribas
Universidad del Rosario, Colombia
 Contribution: processed and analyzed samples, analized data; wrote the manuscript; read and approved the manuscript
Contribution: processed and analyzed samples, analized data; wrote the manuscript; read and approved the manuscript
José Magalhães
Universidade do Porto, Portugal
 Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Edgar Cristancho-Mejía
Universidad Nacional de Colombia, Colombia
 Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Contribution: designed the study and recruited and measured volunteers; analyzed data; wrote the manuscript; read and approved the manuscript
Received: 11 february 2021
Accepted: 09 september 2022
Additional information
How to cite: Ramos-Caballero D M, Rodríguez-Rodríguez P, Mancera-Soto E, Martins S, Arribas Silvia M, Magalhães J, Cristancho-MejíaE. Effect of chronic altitude hypoxia on redox balance in preadolescents and adolescents. Rev Cienc Salud. 2022;20(3):1-16. https://doi.org/10.12804/revistas.urosario.edu.co/revsalud/a.10247
Abstract: Introduction: Living at high altitude increases oxidative stress. Likewise, growth and maturation during adolescence can increase levels of reactive oxygen species (ros). Changes in redox profiles have been evaluated in adults living at high altitudes; however, there are no studies on these changes in peripubertal populations living at moderate altitudes, we determine how living at moderate altitude affects the oxidative and inflammatory status of healthy preadolescents and adolescents. Materials and Methods: A cross-sectional study was conducted in healthy male Colombian preadolescents and adolescents (9–18 years old, Tanner scale classification) who lived at low altitude (n = 26) or moderate altitude (n = 26). Plasma oxidative and inflammatory status was assessed via spectrophotometry. Oxidative markers included malondialdehyde, 4-hydroxy-trans-2-nonenal, and carbonyl groups. Antioxidant markers included total antioxidant status, glutathione, catalase, superoxide dismutase, uric acid, and thiols. Inflammatory markers included interleukins-1, -6, and -10 and tumor necrosis factor. Results: Only uric acid levels were higher in adolescents (5.34 and 5.66 mg/dl) compared to preadolescents (3.85 and 4.07 mg/dl) in both moderate and low altitude groups, respectively. Participants who lived at moderate altitude presented significantly higher levels of malondialdehyde (4.82 and 3.73 nM/mg protein) and lower level of glutathione and thiols (1.21 and 1.26 µmol/mg protein) than in those at low altitude. Their inflammatory profiles did not differ. Conclusion: Oxidant profiles increased in peripubertal populations residing at moderate altitude; this could be owing to antioxidant consumption by ROS and active metabolism during puberty.
Keywords: Oxidative stress, hypoxia, altitude, children, adolescents, redox balance.
Resumen: Introducción: vivir en altura es un factor que se asocia con el estrés oxidativo. El crecimiento y la maduración pueden ser un estresor adicional. Es insuficiente la evidencia sobre alteraciones del perfil redox en peripúberes residentes a altitudes moderadas. El propósito fue establecer el efecto de vivir en una altitud moderada sobre el perfil redox e inflamatorio en preadolescentes y adolescentes sanos. Materiales y métodos: estudio transversal en varones preadolescentes y adolescentes sanos (9-18 años) que viven en altitud baja (n = 26) o altitud moderada (n = 26). El estado oxidativo plasmático se evaluó mediante espectrofotometría a través de marcadores de oxidación (malondialdehído e hidroxinonenal y grupos carbonilo) y antioxidantes (estado antioxidante total, glutatión, catalasa, superóxido dismutasa, ácido úrico y tioles). El perfil inflamatorio se midió con interleucinas 1, 6, 10 y factor de necrosis tumoral α. Resultados: solo el ácido úrico fue diferente entre adolescentes (5.34 y 5.66 mg/dl para moderada y baja altitud, respectivamente) y preadolescentes (3.85 y 4.07 mg/dl para moderada y baja altitud, respectivamente). El grupo de preadolescentes y adolescentes de moderada altitud presentó niveles más altos de malondialdehído (4.82 y 3.73 nM/mg de proteína, respectivamente) y menor glutatión y tioles (1.21 y 1.26 µmol/mg de proteína), en comparación con sus contrapartes de baja altitud. Conclusión: las poblaciones peripúberes que residen en una altitud moderada presentan un perfil oxidante más alto, lo que puede estar relacionado con la depleción de antioxidantes, por una mayor producción de especies reactivas de oxígeno relacionada con la hipoxia y el metabolismo activo de la pubertad.
Palabras clave: estrés oxidativo, hipoxia, altitud, niño y adolescente, oxidación-reducción.
Resumo: Introdução: viver em grandes altitudes é um fator de estresse associado ao estresse oxidativo. Durante a adolescência, os processos de crescimento e maturação podem aumentar as espécies reativas de oxigênio. Alterações no perfil redox foram estudadas em adultos expostos a grandes altitudes, mas não em populações peripubertais vivendo em altitudes moderadas. Nosso objetivo é estabelecer o efeito de viver em uma altitude moderada sobre o estado oxidativo e inflamatório em pré-adolescentes e adolescentes saudáveis. Materiais and métodos: foi realizado um estudo transversal em pré-adolescentes e adolescentes colombianos saudáveis (9-18 anos, na escala de classificação de Tanner) que viviam em baixa altitude (n = 26) ou altitude moderada (n = 26). O estado oxidativo e inflamatório do plasma foi avaliado por espectrofotometria: 1) Marcadores de oxidação: grupos Malondialdeído + 4-hidroxi-trans-2-nonenal e carbonila; 2) antioxidantes: estado antioxidante total, glutationa, catalase, superóxido dismutase, ácido úrico e tióis; 3) Marcadores de inflamação: interleucinas 1, 6, 10 e fator de necrose tumoral α. Resultados: apenas o ácido úrico foi maior em adolescentes (5,34 e 5,66 mg/dl) em comparação com pré-adolescentes (3,85 e 4,07 mg/dl) dos grupos de altitude moderada e baixa, respectivamente. A altitude moderada apresentou níveis significativamente maiores de Malondialdeído (4,82 e 3,73 nM/mg de proteína), e menores níveis de Glutationa e tióis (1,21 e 1,26 µmol/mg de proteína), em comparação com a baixa altitude. Nenhuma diferença foi detectada no perfil inflamatório. Conclusão: as populações peripubertais que residem em altitude moderada apresentam maior perfil oxidante, o que pode estar relacionado ao consumo de antioxidantes devido à maior produção de ROS relacionada à hipóxia e ao metabolismo ativo por volta da puberdade.
Palavras-chave: estresse oxidativo, hipóxia, altitude, crianças, crianças e adolescentes, redução-oxidação.
Introduction
Redox balance determines health. Oxidative stress can disrupt this delicate balance between oxidants and antioxidants by increasing the production of reactive oxygen species (ros), reducing antioxidants, and hindering cellular function, which are associated with pathologies such as cancer and cardiometabolic and respiratory diseases (1). In addition to lifestyle-related factors, environmental influences such as exposure to high altitude hypoxia can affect redox balance. Acute hypoxia triggers electron transport chain complexes to produce greater amounts of ros (2,3). Indeed, inflammatory profiles are linked to oxidative stress, indicating that life at altitude poses stress that could undermine health by disrupting the redox balance (4,5). Studies have shown that the physiological and chemical changes attributed to moderate altitude (MA) are less drastic compared to those attributed to high altitude, though millions of people live at MA (6). In Colombia, nearly 40 % of the population permanently resides at altitudes between 1500 and 3000 m; more than 10 % are children and adolescents under 18 years old (7).
Adults remain the primary subjects in studies that explore how hypobaric hypoxia, or living at high altitude, affects health (8–10). Aging can disrupt redox balance; older adults have greater oxidative stress compared to younger subjects (11). However, critical periods of growth and development during childhood and adolescence may also increase oxidative stress, and specific diseases during infancy have been linked to oxidative damage (12,13).Studies have reported that adolescents have higher levels of oxidants and antioxidants tan children (11,14,15).
The role that oxidative stress plays in disease and the various responses across early stages of life warrant a better understanding of how altitude affects redox balance in younger populations, for whom preventive interventions are possible. Here, we (i) assessed how chronic exposure to ma, or 2000–3000 m above sea level (masl), affects the oxidative profiles of healthy male children and adolescents and (ii) compared the redox statuses of children and adolescents living at ma and low altitude (la) (16). We hypothesized that stress induced by maturation and chronic exposure to hypoxia disrupts the redox balance in children and adolescents living at ma.
Materials and methods
This cross-sectional, descriptive, observational study recruited 52 male children and adolescents between 9 and 18 years of age from two regions in Colombia: 26 residents who lived at la in the city of Tuluá in the Pacific Region (900 masl) and 26 residents who lived at MA in Bogotá (2600–2900 masl). Each subject lived in the corresponding altitude for at least three years and was requested to not travel to a different altitude for three weeks prior to measurements; those who were exposed to a different altitude in that time were excluded. All participants were healthy, did not regularly exercise or play a sport (practice less than six hours per week, corresponding to physical education classes), and had no recent history of infections or treatment with antiinflammatory drugs, antibiotics, or nutritional supplements. All volunteers and their parents or legal guardians consented to this study, which was approved by the research ethics committee of the Science Faculty of the Universidad Nacional de Colombia (Record 18, September 21st, 2016). All participants underwent a medical evaluation and biological maturation assessment according to the Tanner scale to evaluate general health and secondary sexual characteristics, respectively (17). Preadolescents were assigned to stages I and II and adolescents to stages III, IV, and V. Maximal oxygen uptake (VO2 max) was recorded for each participant in his altitude of residence according to a graded exercise test protocol; physical activity levels were assessed by an expert-validated questionnaire on the frequency, duration, and intensity of physical activities that include physical education classes, extracurricular and leisure physical activities, and specific training sessions. Body composition assessed by measuring weight, height, and body fat percentage as described by isak. Body fat percentage was calculated from skinfold measurements using the Slaughter formula (18). A nutritionist noted food frequency and sent 24-hour reminders to detect food ingestion, which could interfere withmeasurements in blood samples.
Redox profiles were measured from blood samples collected at the exercise physiology laboratory at 7:00 a.m. after patients fasted for at least 10 hours and restricted physical activity for 72 hours. An experienced bacteriologist took a blood sample was taken from the antecubital vein by venipuncture in edta vacutainer tubes. The samples were immediately transferred to a refrigerator at 4 °C in the laboratory prior to measuring hematological parameters. The sample was centrifuged at 2400 g for 10 min at 4 °C to separate the plasma, which was stored in aliquots (1 ml) in Eppendorf tubes and subsequently frozen at −80 °C until use. At the same laboratory, the following markers were measured in the plasma samples:
Total antioxidant status (tas). tas was measured with a commercial kit according to the manufacturer´s instructions (nx2332; Randox Laboratories; Crumlin, United Kingdom) with a plate reader (Synergy ht Multimode; BioTek; Winooski, vt, usa).
Reduced glutathione (gsh). gsh levels in breast milk were measured using a fluorometric method based on the o-phthalaldehyde reaction (Hawkins et al. 2008) adapted to a microplate reader (pmid: 32218124). Fluorescence was measured at 360 ± 40 nm excitation and 460 ± 40 nm emission with a microplate reader (Synergy ht Multimode; BioTek; Winooski, vt, usa). gsh was expressed as mg gsh/mL (19).
Uric acid. Uric acid was measured using an enzymatic method according to the manufacturer´s instructions (abx A11A01670; horiba; Montpellier, France).
Thiol content. Plasma thiol levels were quantified using Ellman’s reagent (5,5-dithiobis-2-nitrobenzoic acid, dtnb). Proteins and low-molecular-weight compounds contain thiol groups that react with dtnb and produce 3-nitrobenzoic acid (tnb), which can be quantified at 412 nm with a plate reader (19). Thiol levels were expressed as mM gsh/mL.
Catalase activity (cat). cat was measured by fluorimetry based on the oxidation of the Amplex Red probe produced by H2O2 in the presence of hrp (horseradish peroxidase enzyme). When cat breaks down H2O2 into H2O, it reduces the fluorescence signal, which was measured at an excitation wavelength of 530 ± 25 nm and an emission wavelength of 590 ± 35 nm with a plate reader (19).
Superoxide dismutase (sod). sod activity was measured according to the manufacturer’s instructions (sod Activity Assay kit kb-03-011; Bioquochem; Gijon, Spain) according to the manufacturer´s instructions. Briefly, 20 µL of bm were diluted in deionized H2O (1:1; v/v), followed by adding 200 µL of the working solution and 20 µL of enzyme solution. The reaction was incubated at 37 °C for 20 min. The absorbance was measured at 450 nm with a microplate reader (Synergy ht Multimode; BioTek; Winooski, vt, usa). The sod activity was expressed in percent of inhibition.
Lipid peroxidation. Lipid peroxidation was assessed by measuring the levels of malondialdehyde (mda) and 4-hydroxy-trans-2-nonenal (hne) with a kit (Lipid Peroxidation Assay Kit bm-03-002; Bioquochem; Gijon, Spain) according to the manufacturer´s instructions. Briefly, 100 µL of bm were mixed with 325 µL of chromophore reagent and incubated at 40 °C for 20 min. 200 µL of the reaction or standard curve samples were transferred to a microplate. The absorbance was measured at 586 nm with a microplate reader (Synergy ht Multimode; BioTek; Winooski, vt, usa). Lipid peroxidation was expressed as µM.
Protein oxidation. The levels of carbonylated proteins in bm were measured with a dinitrophenylhydrazine (dnph)-based method adapted to a microplate reader (Synergy ht Multimode; BioTek; Winooski, vt, usa). Absorbance was measured at 370 nm as previously described (14). Carbonyl groups were quantified using the extinction coefficient of 2,4-dinitrophenylhydrazine (ε = 22,000 M/cm) and expressed as nmol/mL.
Plasma cytokines. Plasma levels of proinflammatory cytokines (il-1, il-6, and tnf-α) and antiinflammatory cytokine (il-10) were measured with a cytometric bead array kit (bd Biosciences) and a facsCanto II flow cytometer (bd Biosciences).
Statistical analysis was performed with spss software (version 25.0; ibm; Armonk, ny; usa). The Shapiro-Wilk test was used to assess the normal distribution of the variables, some of which did not follow a normal distribution. A 2 × 2 factorial analysis of variance was used to determine statistical differences between groups. The nonparametric Spearman’s test was used to assess correlations between age and antioxidants and between redox variables and inflammatory markers. The p < 0.05 indicated statistical significance.
Results
A total of 52 volunteers were recruited: 35 were classified as preadolescents (17 residents in LA and 18 residents in ma) and 17 as adolescents (9 residents in la and 8 residents in ma). No statistically significant differences were found in any of the anthropometric param- eters between la and ma residents among preadolescents or adolescents. The age, height, and weight were significantly higher in the adolescent group than the preadolescent group in both settings, but no significant differences were found in bmi, body fat, and VO2 peak (Table 1).
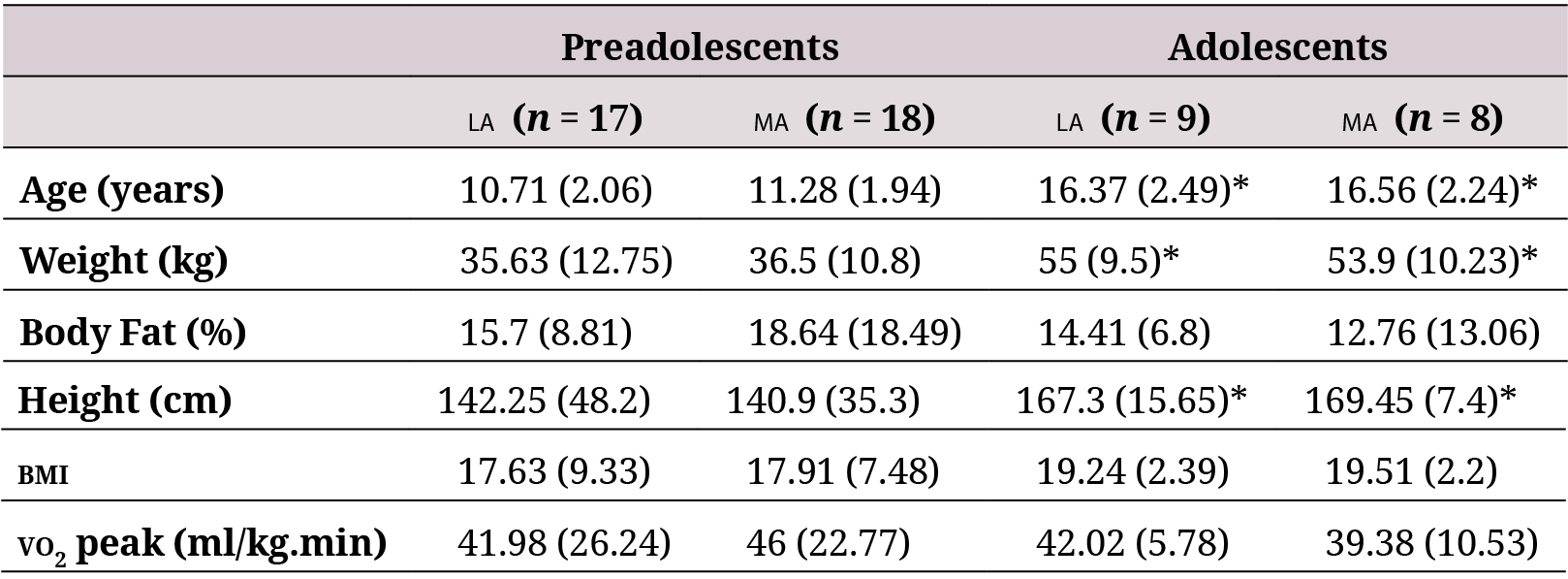
la: low altitude (900 masl). ma: moderate altitude (2600 masl). bmi: Body Mass Index. Data are expressed asmedian (interquartile range).
Altitude affected levels of plasma antioxidants (Figure 1), especially cat (p = 0.011), gsh (p= 0.003), and plasma thiol (p = 0.048). Levels of gsh and plasma thiol of the ma group (pooledpreadolescents and adolescents) were lower than those of the la group (Table 2) by 22.8 %and 16.4 %, respectively.
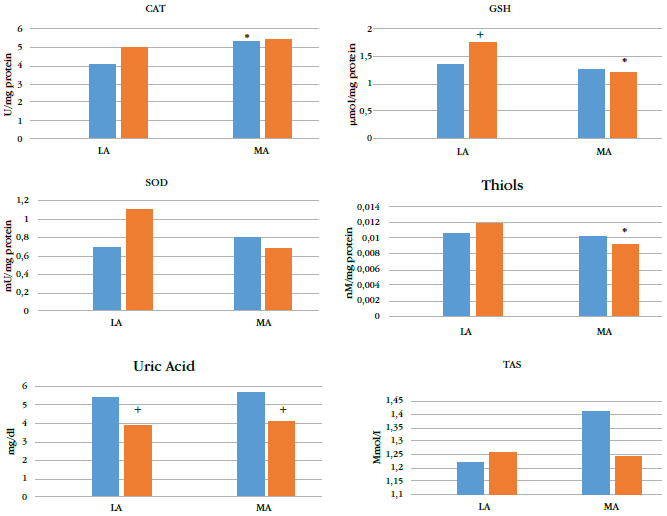
* indicates p < 0.05 for corresponding preadolescents or adolescent group from LA.
+ indicates p < 0.05 for adolescents at the same altitude.
gsh: reduced glutathione; sod: superoxide dismutase; cat: catalase; tas: total antioxidant status; la: low altitude; ma: moderate altitude; bluebars: adolescents; red bars: preadolescents.
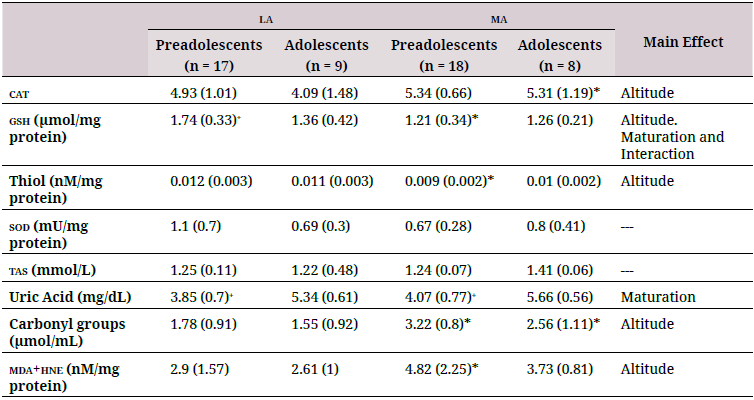
la: low altitude (900 masl); ma: moderate altitude (2600 masl); tas: Total antioxidant status; gsh: reduced glutathione; cat: catalase; sod:superoxide dismutase; mda: malondialdehyde; hne: 4-hydroxy-trans-2-nonenal.
Maturation affected gsh (p = 0.01) and uric acid (p < 0.001) levels). gsh levels in preadolescents living at la were higher than those in adolescents; the opposite was observed in residents at ma, where preadolescents had lower levels of gsh (Table 2). Uric acid levels were higher in adolescents than in preadolescents at both ma and la.
Only gsh levels were significantly affected by an interaction between maturation and altitude (p = 0.037); the ma groups showed lower values. cat, plasma thiol, tas, and uric acid levels were not affected by the interaction between maturation and altitude of residence.
Correlations between age and oxidative status parameters revealed only a moderately positive association between tac and age (p = 0.371) and uric acid and age (p = 0.679). No significant correlations were found among the other variables analyzed (data not shown).
However, altitude significantly affected levels of oxidative damage biomarkers (p = 0.004) (Table 2). The ma group showed significantly higher mda+hne and carbonyl groups compared to the la group (Figure 2). No significant differences were found in oxidation biomarkers to either lipids or proteins between adolescents and preadolescents. There was no significant interaction between maturation and altitude.
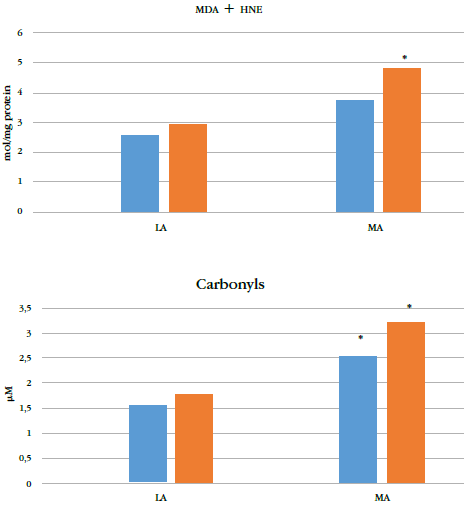
* indicates p < 0.05 for corresponding preadolescents or the adolescent group at la.
Neither altitude, maturation, nor their combination affected the plasma cytokine profile (Table 3). Neither cytokines and antioxidantsnor cytokines and oxidative damage biomarkers strongly correlated with each other (data not shown).
la: low altitude; ma: moderate altitude; hne: 4-hydroxy-trans-2-nonenal.
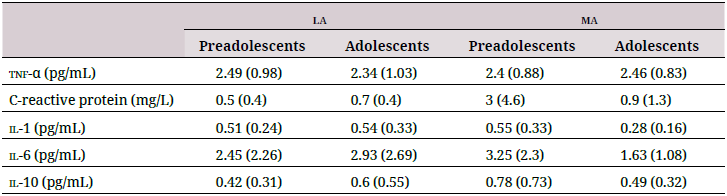
tnf-α: tumor necrosis factor alpha; il-1: interleukin-1; il-6: interleukin-6; il-10: interleukin-10. Data are shown as mean (standard deviation).
Discussion
Several studies have investigated how high altitude (>3500 mas) hypoxia affects oxidative stress in adult populations (9,20–22). However, both ma (2000–3000 masl) and younger populations are often neglected. Here, we explored how living at ma affects the redox profiles of children and adolescents. We found that children and adolescents who live at ma present lower levels of plasma gsh and higher levels of oxidative stress biomarkers. This suggests that chronic exposure to ma during growth and development disrupts the redox balance, which may warrant lifestyle interventions such as diet and exercise to replenish antioxidants.
At high altitude, hypoxia triggers a cascade of signals that can generate ros, whose physiological consequences remain unclear but may induce a hormetic response. On one hand, ros transduce signals and preserve cellular O2 homeostasis by enabling lifelong adaptations to higher altitude (8). However, if produced in excess, ros may amplify oxidative damage(15) that reduces muscle function and capillary perfusion, induces endothelial dysfunction, and contributes to the onset of altitude-related diseases (8). The antioxidant system aims tobalance this redox state.
The impact of high altitude on the oxidative profiles of humans living permanently or intermittingly under such conditions has been evaluated (9,20) However, many people live at ma, whose impact on redox balance in children and adolescents remains largely uncharacterized (21-23). Puberty is a period of rapid growth, development, and maturation and increased anabolic and hormonal activity (24). ROS-activated transduction signals mediate much of this activity (11). Therefore, this population may respond to altitude-induced stress differently from adults.
The antioxidant profiles of individuals acclimatized to high altitude differ across studies; some authors reported low levels of antioxidants following different acclimatization protocols, but others conclude that antioxidant systems adapt to and benefit from life at high altitude (3,10,22,25,26). The latter attributed their findings to molecular mechanisms, particularly the signaling cascades activated by the same ros produced by acute exposure at altitude (26). Duration of residence seems to have an influence: Janocha et al. found that high-altitude residents whose family history spans several millennia of long-term residence at high altitude develop higher defenses than those who descend from populations with only multiple or single generations of residence (9). In the present study, the ma group showed lower levels of some antioxidants, namely thiol groups and gsh. Thiol groups include amino acids (cysteine), some proteins (thioredoxin), and dipeptide gsh, which is the most abundant antioxidant in cells (27). Other studies measured lower levels of gsh at altitude, where hypoxia may disrupt the γ-glutamyl cycle (25). The lower levels of gsh measured in our study may be attributed to hypoxia producing large amounts of ros that consume gsh and other thiol groups (27).
The oxidative statuses of native Andean adults living at 4000 masl and schoolchildren living at 2500 masl in the Guatemalan Highlands have adapted to their environments (28); the levels of antioxidants (retinol, tocopherols, β-carotene and coenzymes Q9 and Q10) were balanced with those of oxidative stress biomarkers (F2-isoprostanes and 8-hydroxy-deoxyguanosine) (29). However, this study did not include lowlanders, and the population was younger (2–7-year-old children) than in our study, which hinders comparisons.
We also analyzed the enzymatic antioxidants sod and cat. How hypobaric hypoxia affectsthese enzymatic systems is debated, but several studies of the Andean region indicated theirlower capacity. Native Bolivian adults exposed to high altitude showed lower levels of catbut higher sod, and adult residents in Huaraz, Peru (3098 masl) showed lower levels of sodcompared to residents at sea level (30). Imai et al. also reported lower antioxidant capacityin an Andean population compared to residents at sea level and attributed this difference tolower levels in glutathione peroxidase (gpx) activity (28). Yet our study found no differencein levels of sod and higher levels of cat.
Previous studies have measured lower levels of antioxidants in children compared to adolescents, suggesting that the antioxidant response matures with age (31). Enzymatic systems may not be fully developed in children and adolescents (32). Children and adolescents undergo significant metabolic and physiological changes due to growth and biological maturation that can increase ros production, which may be accompanied by oxidative stress if levels of endogenous antioxidants are insufficient (14,23,33). Indeed, antioxidant levels negatively correlate with markers of oxidative damage in adolescents and preadolescents (31). Our study found significantly higher levels of tas and uric acid in adolescents compared to preteens as well as a positive correlation between age and levels of these antioxidants regardless of altitude level. Thus, antioxidant capacity may increase with maturation.
The antioxidant levels in the ma population were seemingly insufficient because they were accompanied by higher levels of oxidative damage biomarkers. This is likely related to higher ros production that cannot be balanced by the antioxidant systems. Altitude greater than 2000 masl poses several stress factors: hypoxia, ionizing radiation (infrared and ultraviolet), and cold climates. Each factor can independently increase ros production; their combination together with biological maturation amplifies the oxidizing effect (30). Accordingly, we measured elevated biomarkers of oxidative damage to both lipids and proteins in the moderate-altitude population. Lipids are the first molecules that are susceptible to oxidation; reduced O. levels induce the mitochondrial respiratory chain to increase production of ros and therefore the activity of xanthine oxidase, which causes lipid peroxidation in cell membranes (34). Peroxidation of polyunsaturated fatty acids produces mda, whose levels are higher in adult residents at high altitude as well as in animal models of hypobaric hypoxia (21,30,35). 4-hne is another important signaling molecule that can form covalent adducts with dna, phospholipids, and nucleophilic amino acids, impairing their structure and biological properties (36). Our study supports these findings because we measured higher levels ofprotein carbonyls in children and adolescents exposed to ma.
These results suggest that children and adolescents who live in hypoxic environments lack the adaptive mechanisms of their adult counterparts. The higher levels of carbonyls and lipid peroxidation biomarkers observed at ma in our study could be attributed to both hypoxia and the physiological modifications of peripuberal period. Philips and coworkers reported higher levels of oxidative stress in individuals younger than 20 years old compared to adult subjects (11). Some factors, however, can bolster antioxidant defense systems: Vitamins A, C, and E as well as carotenoids are important dietary nutrients that counteract oxidative stress caused by pro-oxidant like hypoxia to maintain or favor redox balance (37–39). Although none of the participants in this study appeared malnourished according to medical examination and anthropometric characteristics, their dietary patterns and levels of exogenous antioxidants should be monitored. Whether antioxidant supplements are beneficial at high altitude also remains controversial (40). In this study, VO2 max, an indicator of cardiorespiratory fitness, did not differ across groups, which suggested that levels of physical activity did not contribute to differences in oxidative profiles. Future studies should include children who are more physically active in the same settings because the antioxidant systems in children and adolescents exhibit benefit from exercise (41). How exercise affects redox balance in children and adolescents who live at ma remains unknown.
Many studies have associated oxidative stress with inflammation. A systemic inflammatory response produces greater amounts of cytokines in the blood in response to hypoxia, which activates tissue damage-repair mechanisms mediated by the coactivation of immune and endothelial cells (42). Indeed, acute exposures to altitude can induce chronic responses (43). Other factors such as training can influence this inflammatory response (3). However, cytokines and oxidative stress markers were not correlated in the young population we studied. This suggests that pro-oxidants in younger individuals who live at altitude do not trigger inflammatory responses, which may indicate a better adaptation. This supports a study on schoolchildren living at ma, whose levels of pro- and antiinflammatory cytokines were correlated and suggested an adaptation that protects against inflammation at altitude (29).
In this study, moderate altitudinal hypoxia disrupted the redox balance in male children and adolescents. This could be due to lower antioxidant levels, higher ros production, and active metabolism during growth and development. We evaluated only male subjects because it was impossible to test hormonal parameters, which could interfere with the study. However, future work should include female subjects to determine if gender also plays a role. A larger sample size would also help elucidate the varied responses to oxidative stress among children and adolescents. Finally, our data introduce the opportunity to perform an intervention study to determine if antioxidants or sport counteract altitude-induced oxidativestress in young populations.
Acknowledgments
We thank the following institutions and entities for their support in enrolling participants and allowing our access to facilities: Unidad Central del Valle, Tuluá, Colombia; Unit of Applied Sciences to Sports (ucad) of the District Institute of Recreation and Sports (idrd), Bogotá, Colombia; Laboratory of Doping Control, Bogotá, Colombia; School Instituto Pedagógico Arturo Ramirez Montufar of Universidad Nacional de Colombia, Bogotá, Colombia.
Author contributions
Diana Marcela Ramos-Caballero, Erica Mancera-Soto, José Magalhães, and Edgar Cristancho-Mejía designed the study and recruited and measured volunteers. Pilar Rodríguez-Rodríguez, Silvia M. Arribas, and Sandra Martins processed and analyzed samples. All authors analyzed data and wrote the manuscript. All authors read and approved the manuscript. All persons designated as authors qualify for authorship, and all who qualify forauthorship are listed.
Funding
This research was supported by Red iberoamericana de Medicina y Fisiología de altura (cyted; 213-rt0478 to Edgar Cristancho, José Magalhaes, and Silvia M. Arribas) and internal sources from Universidad Nacional de Colombia (Edgar Cristancho), Universidade do Porto (José Magalhaes); Universidad Autónoma de Madrid (Silvia M. Arribas).
Conflict of interests
None declared.
References
1. Lichtenberg D, Pinchuk I. Oxidative stress, the term and the concept. Biochem Biophys Res Commun. 2015;461(3):441-4. https://doi.org/10.1016/j.bbrc.2015.04.062
2. Fuhrmann DC, Brüne B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017;12:208-15. https://doi.org/10.1016/j.redox.2017.02.012
3. McGarry T, Biniecka M, Veale DJ, Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med 2018;125:15-24. https://doi.org/10.1016/j.freeradbiomed.2018.03.042
4. Pillon Barcelos R, Freire Royes LF, Gonzalez-Gallego J, Bresciani G. Oxidative stress and inflammation: liver responses and adaptations to acute and regular exercise. Free Radic Res. 2017;51(2):222-36. https://doi.org/10.1080/10715762.2017.1291942
5. Stöwhas AC, Latshang TD, Lo Cascio CML, Lautwein S, Stadelmann K, Tesler N, et al. Effects of acute exposure to moderate altitude on vascular function, metabolism and systemic inflammation. PLoS ONE. 2013;8(8):e70081. https://doi.org/10.1371/journal.pone.0070081
6. Brothers MD, Doan BK, Zupan MF, Wile AL, Wilber RL, Byrnes WC. Hematological and physiological adaptations following 46 weeks of moderate altitude residence. High Alt Med Biol. 2010;11(3):199-208. https://doi.org/10.1089/ham.2009.1090
7. dane/Colombia. Censo nacional de población y vivienda (cnpv); 2018 [cited 2020 Dec 18]. Available from: http://microdatos.dane.gov.co/index.php/catalog/643/get_microdata
8. Bailey DM. Oxygen, evolution and redox signalling in the human brain; quantum in the quotidian. J Physiol. 2019;597(1):15-28. https://doi.org/10.1113/JP276814
9. Janocha AJ, Comhair SAA, Basnyat B, Neupane M, Gebremedhin A, Khan A, et al. Antioxidant defense and oxidative damage vary widely among high-altitude residents. Am J Hum Biol. 2017;29(6). https://doi.org/10.1002/ajhb.23039
10. Magalhães J, Ascensão A, Marques F, Soares JM, Ferreira R, Neuparth MJ, et al. Effect of a high-altitude expedition to a Himalayan peak (Pumori, 7,161 m) on plasma and erythrocyte antioxidant profile. Eur J Appl Physiol. 2005;93(5-6):726-32. https://doi.org/10.1007/s00421-004-1222-2
11. Phillips M, Cataneo RN, Greenberg J, Gunawardena R, Rahbari-Oskoui F. Increased oxidative stress in younger as well as in older humans. Clin Chim Acta. 2003;328(1-2):83-6. https://doi.org/10.1016/S0009-8981(02)00380-7
12. Kogawa T, Nishimura M, Kurauchi S, Kashiwakura I. Characteristics of reactive oxygen metabolites in serum of early teenagers in Japan. Environ Health Prev Med. 2012;17(5):364-70. https://doi.org/10.1007/s12199-011-0261-7
13. Granot E, Kohen R. Oxidative stress in childhood--in health and disease states. Clin Nutr. 2004;23(1):3-11. https://doi.org/10.1016/S0261-5614(03)00097-9
14. Pérez-Navero JL, Benítez-Sillero JD, Gil-Campos M, Guillén-del Castillo M, Tasset I, Túnez I. Changes in oxidative stress biomarkers induced by puberty. An Pediatr (Barc). 2009;70(5):424-8. https://doi.org/10.1016/j.anpedi.2009.01.019
15. Tsukahara H. Biomarkers for oxidative stress: clinical application in pediatric medicine. Curr Med Chem. 2007;14(3):339-51. https://doi.org/10.2174/092986707779941177
16. Bärtsch P, Saltin B. General introduction to altitude adaptation and mountain sickness. Scand J Med Sci Sports. 2008;18;Suppl 1:1-10. https://doi.org/10.1111/j.1600-0838.2008.00827.x
17. Tanner JM. The measurement of maturity. Trans Eur Orthod Soc. 1975:45-60.
18. Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60(5):709-23.
19. Ramiro-Cortijo D, Calle M, Rodríguez-Rodríguez P, Pablo ÁLL, López-Giménez MR,Aguilera Y et al. Maternal antioxidant status in early pregnancy and development offetal complications in twin pregnancies: a pilot study. Antioxidants (Basel). 2020;9(4). https://doi.org/10.3390/antiox9040269
20. Askew EW. Work at high altitude and oxidative stress: antioxidant nutrients. Toxicology. 2002;180(2):107-19. https://doi.org/10.1016/s0300-483x(02)00385-2
21. Pomar IGM, Erquicia EB, Colmena LT, Barrera SQ, Vargas PLS. Concentration of malondialdehyde in subjects living at high altitudes: exploratory study. Rev Peru Med Exp Salud Publica. 2017;34(4):677-81. https://doi.org/10.17843/rpmesp.2017.344.2830
22. Sinha S, Ray US, Tomar OS, Singh SN. Different adaptation patterns of antioxidant systemin natives and sojourners at high altitude. Respir Physiol Neurobiol. 2009;167(3):255-60. https://doi.org/10.1016/j.resp.2009.05.003
23. Soto-Méndez MJ, Romero-Abal ME, Aguilera CM, Rico MC, Solomons NW, Schümann K, et al. Associations among inflammatory biomarkers in the circulating, plasmatic, salivaryand intraluminal anatomical compartments in apparently healthy preschool childrenfrom the Western Highlands of Guatemala. Plos One. 2015;10(6):e0129158. https://doi.org/10.1371/journal.pone.0129158
24. Zalavras A, Fatouros IG, Deli CK, Draganidis D, Theodorou AA, Soulas D, et al. Age-related responses in circulating markers of redox status in healthy adolescents and adults duringthe course of a training macrocycle. Oxid Med Cell Longev. 2015;2015:283921. https://doi.org/10.1155/2015/283921
25. Bakonyi T, Radak Z. High altitude and free radicals. J Sports Sci Med. 2004;3(2):64-9.
26. Magalhães J, Oliveira J, Ascensão A. Oxidative stress in high-altitude hypoxia. Is it truly a paradox? Muscle Plast Adv Biochem Physiol Res. 2009:121-50.
27. Oliveira PVS, Laurindo FRM. Implications of plasma thiol redox in disease. Clin Sci (Lond). 2018;132(12):1257-80. https://doi.org/10.1042/CS20180157
28. Imai H, Kashiwazaki H, Suzuki T, Kabuto M, Himeno S, Watanabe C, et al. Selenium levels and glutathione peroxidase activities in blood in an Andean high-altitude population. J Nutr Sci Vitaminol (Tokyo). 1995;41(3):349-61. https://doi.org/10.3177/jnsv.41.349
29. Soto-Méndez MJ, Aguilera CM, Mesa MD, Campaña-Martín L, Martín-Laguna V, Solomons NW, et al. Strong associations exist among oxidative stress and antioxidant biomarkers in the circulating, cellular and urinary anatomical compartments in Guatemalan children from the Western Highlands. Plos One. 2016;11(1). https://doi.org/10.1371/journal.pone.0146921
30. Ramón C, Dora J, Villavicencio Villanueva JN. Indicadores de estrés oxidativo en eritrocitos de una población de Huaraz; 2013.
31. Paltoglou G, Fatouros IG, Valsamakis G, Schoina M, Avloniti A, Chatzinikolaou A, et al. Antioxidation improves in puberty in normal weight and obese boys, in positive association with exercise-stimulated growth hormone secretion. Pediatr Res. 2015;78(2):158- 64. https://doi.org/10.1038/pr.2015.85
32. Maciejczyk M, Zalewska A, Ładny JR. Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxid Med Cell Longev. 2019;2019:4393460. https://doi.org/10.1155/2019/4393460
33. Cooper DM, Nemet D, Galassetti P. Exercise, stress, and inflammation in the growing child: from the bench to the playground. Curr Opin Pediatr. 2004;16(3):286-92. https://doi.org/10.1097/01.mop.0000126601.29787.39
34. Prijanti AR, Iswanti FC, Ferdinal F, Jusman SWA, Soegianto RR, Wanandi SI, et al. Hypoxia increased malondialdehyde from membrane damages is highly correlated to HIF-1α but not to renin expression in rat kidney. IOP Conf Ser Earth Environ Sci. 2019;217:012062. https://doi.org/10.1088/1755-1315/217/1/012062
35. Catherine C, Ferdinal F. Effect of chronic systemic hypoxia on malondialdehyde (MDA) levels in blood and kidney tissue of Sprague Dawley rats; 2019.
36. Xiao M, Zhong H, Xia L, Tao Y, Yin H. Pathophysiology of mitochondrial lipid oxidation: role of 4-hydroxynonenal (4-hne) and other bioactive lipids in mitochondria. Free Radic Biol Med. 2017;111:316-27. https://doi.org/10.1016/j.freeradbiomed.2017.04.363
37. Fang Y-Z, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18(10):872-9. https://doi.org/10.1016/S0899-9007(02)00916-4
38. McLeay Y, Stannard S, Houltham S, Starck C. Dietary thiols in exercise: oxidative stress defence, exercise performance, and adaptation. J Int Soc Sports Nutr. 2017;14:12. https://doi.org/10.1186/s12970-017-0168-9
39. Zanella PB, August PM, Alves FD, Matté C, de Souza CG. Association of Healthy Eating Index and oxidative stress in adolescent volleyball athletes and non-athletes. Nutrition. 2019;60:230-4. https://doi.org/10.1016/j.nut.2018.10.017
40. Stellingwerff T, Peeling P, Garvican-Lewis LA, Hall R, Koivisto AE, Heikura IA, et al. Nutrition and altitude: strategies to enhance adaptation, improve performance and maintain health: a narrative review. Sports Med. 2019;49(Suppl 2):169-84. https://doi.org/10.1007/s40279-019-01159-w
41. Avloniti A, Chatzinikolaou A, Deli CK, Vlachopoulos D, Gracia-Marco L, Leontsini D, et al. Exercise-induced oxidative stress responses in the pediatric population. Antioxidants (Basel). 2017;6(1). https://doi.org/10.3390/antiox6010006
42. Hartmann G, Tschöp M, Fischer R, Bidlingmaier C, Riepl R, Tschöp K, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246-52. https://doi.org/10.1006/cyto.1999.0533
43. Al-Hashem FH, Assiri AS, Shatoor AS, Elrefaey HM, Alessa RM, Alkhateeb MA. Increased systemic low-grade inflammation in high altitude native rats mediated by adrenergic receptors. Saudi Med J. 2014;35(6):538-46.
Author notes
* Correspondence author: diana.ramos@urosario.edu.co



