Background
Worldwide, asthma is recognized as the most prevalent chronic respiratory disease, affecting approximately 334 million people. It ranks as the 14th most significant disease in terms of prevalence and duration of disability 1. Asthma is characterized by chronic airway inflammation accompanied by airflow obstruction, leading to intermittent respiratory muscle load and muscle overload during acute exacerbations 2,3. This muscle dysfunction is evidenced by decreased muscle strength and reduced net excitation of inspiratory motor neurons 4,3.
Understanding the functional state of respiratory muscles is crucial to determine the functional consequences and level of asthma control. Consequently, various technological strategies have been developed to assess respiratory muscle function, including measurements of maximal inspiratory and expiratory pressures (MIP and MEP), spirometry, and electromyography 5-7. However, these procedures are not always available in clinical settings. As an alternative, monitoring breathing patterns, costal mobilization, and muscle contraction through palpation are traditionally part of Manual Muscle Testing (MMT) 5. MMT has been recognized as a simple, cost-effective, and accessible tool 8. Despite its advantages, the psychometric properties of MMT have primarily been evaluated on limb muscles, as evidenced in the literature 9.
The availability of reliable and valid measurement tools in clinical practice allows for unbiased assessments, supports diagnoses, and facilitates the implementation of treatments aimed at improving ventilatory mechanics compromised by asthma 3. Moreover, in research, these tools enhance the reliability of physiotherapeutic intervention outcomes by minimizing the likelihood of measurement variability.
Accordingly, it is essential to assess the psychometric properties of tools used in clinical practice and compare them with validated and reliable tests. In this context, the reliability and validity of MMT compared to MIP and MEP should be evaluated. This approach would complement ventilatory function assessment in patients with asthma, enabling better disease monitoring, supporting therapeutic programs to control symptoms, preventing complications, and improving overall function and quality of life in this population 2. Therefore, the research question of this study was: What is the intra- and interrater reliability of MMT for the diaphragm, external intercostal, and abdominal muscles in a population of patients with asthma? What is the convergent validity of MMT compared to MIP and MEP in a population of patients with asthma?
Methods
he evaluation of diagnostic test reliability and validity was conducted using a cross-sectional sampling method 10).
Subjects. The study included adults with asthma in stable phases of the disease, as defined by the Global Initiative for Asthma (GINA) criteria, which consider the control of signs and symptoms and functional test results 2. Patients were excluded if they had comorbidities such as heart disease, uncontrolled arterial hypertension, recent lung biopsy, spinal cord injury, ocular lesions, tracheotomy, upper airway surgery or trauma, hemodynamic instability, pregnancy, or respiratory infections 11. Additionally, patients with musculoskeletal or neurological sequelae compromising thoracic mobility and muscle control were excluded, as were those who demonstrated a lack of voluntary effort during spirometry 12, defined by a Peak Expiratory Flow (PEF) or Forced Expiratory Flow at 25 % (FEF25%) below 60% of the predicted value 13. Measurements were conducted in the Laboratory of Movement Analysis at the School of Physiotherapy, Universidad Industrial de Santander.
Evaluators. Two clinically experienced physiotherapists participated in the study. They standardized verbal instructions and hand placements and were trained in administering the tests to avoid classification bias.
Procedures
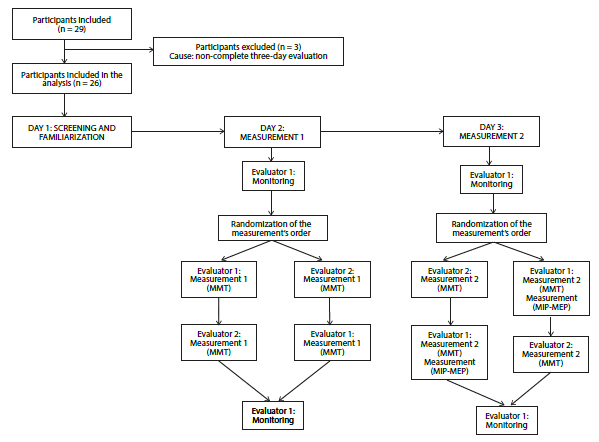
The protocol included measurements conducted over three days, spaced 2 and 8 days apart. On Day 0, screening and familiarization were carried out. During this session, anthropometric, spirometric, sociodemographic, disease-related, and monitoring-related variables were measured. Additionally, participants were familiarized with the muscle strength assessment process. On the second day, MMT was independently measured by two evaluators. On the third day, MMT was measured again by the same evaluators. One evaluator also measured Maximal Inspiratory and Expiratory Pressures (MIP and MEP) during these sessions. All variables were measured in a randomized order. Vital signs were monitored at the beginning and end of each session by one evaluator. Both evaluators were blinded to previous measurements and to each other's results. To ensure consistency, evaluations were conducted at the same time each day. Participants were instructed to continue their medical treatments throughout the study (Figure 1).
Measurements
Spirometry. Spirometry was performed using a Spirobank G (MIR SRL) device, adhering to the technical standards established by the American Thoracic Society and the European Respiratory Society 6,13. Subjects performed at least three maximal forced expiratory maneuvers, with PEF and FEF25% were recorded. Participants with results below 60% of the predicted value were excluded from the study 6,13. Additionally, forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio were recorded.
Anthropometric variables. Body weight (kg) was measured using a portable, digital, and calibrated scale. Height (m) was measured with an inextensible metric tape with 1 mm precision. The Body Mass Index (BMI = weight/height2) was then calculated. All measurements followed the guidelines outlined in the Anthropometry Procedures Manual of the National Health and Nutrition Examination Survey by the Centers for Disease Control and Prevention 14.
Monitoring variables. Heart rate (beats/min), respiratory rate (respirations/min), blood pressure (mmHg), and oxygen saturation (SaO2, percentage) were recorded with a precision of ±2%. Additional data, including lung auscultation findings, breathing difficulty (assessed using the Borg Scale), and signs of respiratory distress, were also documented 15,16.
Variables of muscle strength. Muscle strength was assessed through MMT and static respiratory pressure measurements. MMT was conducted using palpation, thoracic mobility observation, and manual resistance applied against contracting muscles, based on the ordinal grading system from the Medical Research Council (MRC) 17. The protocol outlined evaluator and participant positioning, hand placement for muscle palpation, palpation pressure, and verbal commands. For inspiratory and expiratory muscles, the grading scales differed: a "normal /good /fair /poor /trace /null" scale was used for the diaphragm and external intercostal muscles, while "functional /slightly functional /non-functional /null" was applied to abdominal muscles, based on coughing mechanisms 8,18. The evaluator initiated the test by issuing a verbal command and observing muscle contraction. Hands were then placed on specific muscle points for palpation without resistance, followed by the application of manual resistance against muscle movement 8,18. Each muscle was tested twice, with a one-minute interval between attempts, and the higher value was recorded.
MIP and MEP were measured using a manometer equipped with a nozzle and valves to sense pressure changes. MIP was defined as the maximal subatmospheric pressure generated against an occluded airway from residual volume (Müller maneuver), sustained for one second. MEP was measured at total lung capacity (TLC), recording the maximal expiratory pressure against an occluded airway (Valsalva maneuver) for one second 19.
Statistics. A sample size of25-35 participants was determined as appropriate for assessing MMT psychometric properties. This calculation considered the use of two replicates, with an 80% statistical power, a 5% significance level, and a 20% expected loss rate 20.
Measures of central tendency and dispersion were calculated to describe the population, considering the nature and distribution of variables. Intra- and inter-rater reliability of MMT was evaluated using weighted kappa 10. Landis and Koch guidelines 21 were applied to interpret kappa values: slight (kappa = 0.0-0.2), fair (kappa = 0.21-0.40), moderate (kappa = 0.41-0.60), substantial (kappa = 0.61-0.80), and almost perfect (kappa = 0.81-1.00) agreement. Convergent validity between MMT and static pressures was assessed using the Pearson correlation coefficient. Correlation strength was classified based on Carter and Lubinsky 22: small (r = 0.0-0.25), low (r = 0.26-0.49), moderate (r = 0.50-0.69), high (r = 0.70-0.89), and very high (r = 0.90-1.00).
Results
Flow of participants. Twenty-nine adults with stable asthma were initially enrolled in the study. However, three participants did not complete the scheduled evaluations, leaving 26 patients included in the final analysis (Figure 1). Of these, 16 (61.54%) were female. Table 1 summarizes the general, disease-related, and spirometric characteristics of the patients. All participants demonstrated normal spirometric patterns, as indicated by FEV1 and FVC values, with FEV1/FVC ratios exceeding 80% and 70% of predicted values 6.
Table 1
Characteristics of sample (n = 26)
Reliability of MMT. Intrarater reliability was comparable for both evaluators. Agreement levels were substantial (kappa = 0.78-0.88) for the superior external intercostal and abdominal muscles, and moderate (kappa = 0.45-0.59) for the anterior and posterior diaphragm. Wide confidence intervals were observed for all evaluated muscle groups. These findings are detailed in Table 2.
Table 2
MMT intrarater reliability (n = 26)
Interrater reliability ranged from slight to substantial during the evaluations conducted on the second and third days. While confidence intervals remained wide, they were narrower for the lateral diaphragm. Table 3 presents the interrater reliability results for each muscle group.
Table 3
MMT interrater reliability (n = 26)
Convergent validity of mmt and static respiratory pressures. The correlation between static respiratory pressures and mmt was generally small to low for both inspiratory and expiratory muscles (Table 4).
Table 4
Correlation between static respiratory pressures and MMT (n = 26)
Discussion
The intrarater reliability of MMT was substantial for the diaphragm, external intercostal, and abdominal muscles. These results likely reflect the rigorous standardization of the protocol used in the study. Unfortunately, no prior studies have evaluated the reliability of MMT for respiratory muscles, precluding direct comparisons 9,23,24.
Reliability levels varied within muscle groups, ranging from almost perfect to moderate. These differences cannot be attributed to changes in muscle strength, as the short interval between measurements was insufficient to produce significant changes. Instead, they may be explained by the biomechanics of respiratory muscles. The almost perfect agreement observed for the superior external intercostal muscles and the substantial reliability for abdominal muscles likely stem from their superficial anatomical location, which facilitates palpation 18,25,26.
Moderate to substantial reliability for the diaphragm requires consideration of its unique function and anatomical complexity. When the diaphragm contracts, its central tendon descends, and the dome flattens complicating its palpation during testing 18,26,27. Additionally, chronic respiratory diseases can pathologically flatten the diaphragm, altering its function. This reduces thoracic expansion and may impair performance during evaluations, presenting challenges for evaluators 25,28. Lower reliability in the inferior external intercostal muscles may be linked to interference from diaphragm activity. The diaphragm's action depends on its attachment to the six lower ribs and the zone of apposition, where it is juxtaposed to the costal margin's inner surface 28.
The overall interrater reliability is influenced by the evaluators' training and standardization of the measurement protocol. Although these factors were controlled in the present study, the evaluated construct (muscle strength) by MMT involves a subjective assessment. Consequently, the variability in the observations can be attributed to differences in the resistance applied to the muscle and the grading of the perceived performance.
While hand contact was standardized, manual resistance was not quantified, potentially leading to differences in force application between evaluators. Such variability could particularly affect the external intercostal muscles, as rib forces are transmitted through rib cage joints 25,29,30. The fair interrater reliability observed for abdominal muscles during the first evaluation may stem from the grading scale's limited differentiation between "functional" and "weakly functional" levels, leading to inconsistencies in evaluator criteria.
When comparing measurements of the two days, interrater reliability was greater in the second measurement for most muscles (Table 3). This can be attributed to the learning effect of participants during test evaluation. In this context, Lavietes et al. 31 described a training-derived outcome when repeated measurements of MIP were done in adults with acute asthma. Possibly the same factor is present in MMT since both methods assess respiratory muscle strength. Therefore, in every new measurement, muscle performance improves as a result of previous experience.
The confidence intervals of kappa coefficients calculated for both days were wide, reflecting the typical variability of the construct evaluated and the subjectivity of MMT in respiratory muscles. Measurement variability can be explained by the characteristics of these muscle groups, such as their localization within the thoracic cavity 18,25,27 and the influence that costal and pulmonary biomechanics may exert on them 12. The strength of muscle contraction is determined by muscle length-tension, force-velocity, and stimulating strength-frequency relationships, as well as by the integrity of the contractile apparatus 27. Therefore, small changes in these factors may result in variations in muscle strength perceived through palpation.
Reliability variations in diaphragm fiber data could be influenced by the extent of abdominal muscle relaxation, posture during testing, and modifications in ventilatory mechanics 8,18,25. For external intercostal muscles, the test relies on the degree of horizontal and vertical movement of the ribs, changes in the intercostal spaces, the expansion of the chondrosternal angle during the breathing cycle, and diaphragm relaxation. Variations in these factors increase measurement variability. Additionally, abdominal muscles are evaluated considering their ability to generate expiratory flows and their location within the thoracic wall 8,18,32.
Beyond physiological characteristics contributing to variability, it is important to mention the sample size. Although the calculated sample size was achieved, it may not have been sufficient to ensure greater accuracy in the reliability measurements.
The convergent validity of MMT and static respiratory pressures was low. Correlation analysis was performed between MIP and the average score of diaphragms and external intercostal muscles, as well as between MEP and the average score of abdominal muscles. However, the analysis was not performed specifically for each subgroup of muscles, as respiratory pressures do not differentiate strength by muscle group 19. For this reason, and for the reasons discussed below, MIP and MEP may not serve as the best comparison standards.
The low overall correlation should be analyzed considering the measurement protocols for both variables. Patients for MMT are assessed in a supine position, whereas pressures are measured with the patient seated. Posture affects the performance of respiratory muscles, particularly the inspiratory muscles, which work against resistance to overcome the elasticity of the rib cage, the elastic resistance of the lungs, gravity, and abdominal contents 18,27.
Recruitment of respiratory muscles depends on breathing type, posture, and thoracic wall characteristics 18,27. The work of the diaphragm and intercostal muscles can be observed in different positions. In a vertical posture, during inhalation, the diaphragm pulls the phrenic center downward, increasing the thoracic cavity vertically. During exhalation, the diaphragm relaxes, its dome lifts, and the thoracic volume decreases. In the decubitus position, used for MMT, the diaphragm continues to function according to the pressures it receives. Specifically, in the lateral decubitus position, the hemidiaphragm on the lying side is pushed by intra-abdominal pressure and has a more expiratory role than in other positions 29. he work of intercostal muscles is influenced by rib posture, as the forces produced by these muscles are transmitted through joints and cartilage to other bones 18,25,27.
The expiratory action of abdominal muscles can be explained by trunk position, as flexion facilitates air expulsion 18, and by the specificity of muscle activation. At the end of inspiration and the onset of expiration, the muscles most activated -such as the minor oblique, transversus abdominis, and lateral fibers of the major oblique -are the least involved in trunk flexion 18. Additionally, in standing and sitting postures, the diaphragm's inspiratory action is complemented by abdominal muscle tension, which facilitates an increase in abdominal pressure and prevents abdominal protrusion during inspiration 25.
Another difference between the maneuvers for testing respiratory pressures and MMT was the resistance applied. The manometer used for evaluating pressures provides an occlusion to the airflow, imposing a fixed resistance to muscle work 19, whereas in MMT, the evaluator manually applies resistance that is not quantified. The low correlation between static respiratory pressures and MMT can also be attributed to the activation of muscle groups during the maneuvers. The effort measured through MIP results from the joint activation of inspiratory muscles 33, while MMT discriminates the effort of each muscle group. Therefore, the low correlation could highlight an advantage of MMT over the measurement of respiratory pressures. Unfortunately, this advantage could not be corroborated in the present study because electromyographic activity was not evaluated.
A constraint in the study was its restriction to adults with stable asthma, as the psychometric properties of MMT were established only for such patients. It is recommended that psychometric properties be evaluated across different asthma phases, age groups, and other pathologies. Another limitation of the study was that the manual resistance applied by evaluators during MMT was not quantified. Although this parameter can be measured precisely through dynamometry, applying this measurement to respiratory muscles (particularly the intercostals) is challenging due to their anatomical disposition. Additionally, the use of dynamometry in clinical practice is restricted because of limited equipment availability. Muscle strength data measured by static respiratory pressures is also difficult to compare with other studies due to the use of different scales. Consequently, reporting the measured effort as a percentage is advisable, as it facilitates comparison across studies.
Accordingly, it can be concluded that MMT is a reliable method for evaluating the respiratory muscle strength of patients with asthma. This assessment can be conducted by health professionals during thoracic physical examinations, broadening the analysis of ventilatory mechanics in each case. Despite its subjectivity, it is a useful, practical, low-cost, and easy-to-perform tool for assessing muscle groups and differentiating their fibers. When correctly applied, MMT is an efficient procedure within the clinical assessment of muscle function, provided that the basic conditions for test efficacy are met. These include evaluator-specific training, relaxation of adjacent musculature, proper posture, adequate hand placement, and standardized verbal commands.
Conclusions
Intra- and interrater reliability for MMT ranged from substantial to moderate, except for inter-rater reliability for the superior external intercostal muscles on the second day of measurements, which was graded as slight. Correlations between MMT and MIP, as well as MEP, were low.
Results from this study support the application of MMT for respiratory muscles in clinical settings when more objective measures, such as MIP and MEP, are not available. Despite its subjectivity, it is a useful, practical, low-cost, and easy-to-perform tool for assessing muscle groups and differentiating their fibers, provided that the basic conditions for the test's efficacy are met. These conditions include evaluator-specific training, relaxation of adjacent musculature, proper posture, adequate hand placement, and standardized verbal commands to facilitate touch sensitivity for muscle contraction.
Accordingly, it can be concluded that MMT is a reliable method for evaluating the respiratory muscle strength of patients with asthma. Further studies could evaluate its convergent validity compared with dynamometry or electromyography of the respiratory muscles.